Introduction
Bruton's tyrosine kinase inhibitors (BTKi) have improved 1 st line therapy efficacy in elderly (Wang NEJM 2022) and young, fit, MCL-the latter with intermittent dosing during RCHOP in an alternating RCHOP/cytarabine-based regimen (Dreyling Blood 2022). However direct combination of BTKi & intensive chemo is toxic in DLBCL studies. (Kuruvilla Haematol Oncol 2017). The highly selective BTKi, acalabrutinib (A), has proven efficacy and tolerability in relapsed MCL (Wang Lancet 2018). RDHAOx chemotherapy (rituximab, dexamethasone, cytarabine, oxaliplatin) prior to ASCT provides a complete response (CR) rate of 77% and favourable toxicity compared to many induction regimens (Le Gouill Blood 2017). Furthermore, Obinutuzumab-DHAP yields undetectable minimum residual disease (MRD) in 85% of young MCL patients. (Le Gouill Haematol Oncol 2019). Here we report the primary endpoint from the Australasian Leukaemia & Lymphoma Group phase 2 ‘WAMM’ trial exploring a ‘sandwich’ model of an acalabrutinib-rituximab (AR) ‘window’ before RDHAOx +/- ASCT, followed by fixed-duration AR-maintenance to improve therapy response and minimise additional toxicity.
Methods
NHL33'WAMM' (ACTRN12619000990123) is a multicentre single-arm phase 2 trial. Key eligibility included: age 18-70 years, untreated histologically-proven stage II-IV MCL, ECOG<2; no contraindications to ASCT or BTKi. Pts received 2 cycles of AR (AR window); ‘A’ dose; 100mg BD PO, ‘R’ dose; 375mg/m 2 IV (day 1) every 4 weeks, followed by RDHAOx x4 cycles. Those with an objective response (CR or partial response-PR) underwent BEAM ASCT (carmustine, etoposide, cytarabine, melphalan) then AR maintenance (A; 1yr continuous & R; 3-monthly x 8 cycles). Those who did not undergo BEAM ASCT were able to remain on study and proceed to maintenance AR in the event of ongoing response. Co-primary endpoints (EP) were safety; defined by lack of prohibitive toxicity causing treatment cessation AND PET-determined complete response (CR) rate after AR+RDHAOx. Secondary EP include overall response rates (ORR), toxicity, overall survival (OS) & progression-free survival (PFS), MRD negativity rates and quality of life. Only Grade 3+ toxicity related to acalabrutinib was collected during the ASCT period. Baseline whole exome sequencing was performed to identify mutations, MRD analysis was done by LymphoTrack® Dx and MRD Assay platform (Invivoscribe, Inc) using Illumina® MiSeq. This study was the first Australian blood cancer trial to use telehealth and a ‘hub-and-spoke’ transplant model.
Results
44 pts were enrolled from Sept 2020 to Apr 2022 (43 evaluable for the primary endpoint). Baseline characteristics were typical of a young MCL cohort: median age 59 years (Interquartile range 54-64), 77% were male, ECOG was 0-1 in 98%, 84% had stage IV, lactate dehydrogenase was elevated in 35%, Ki-67 >30% in 66% and blastoid/pleomorphic histology 9%. TP53 by NGS will be reported at presentation.
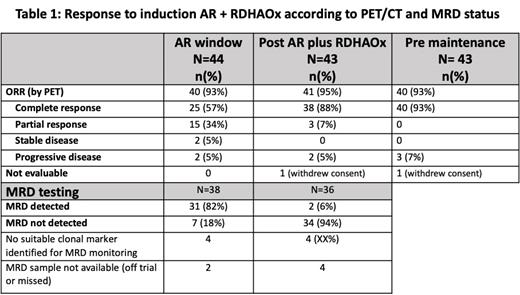
CR rate post AR+RDHAOx induction was 88%; (95%CI 72-95) with ORR 95% and no prohibitive toxicity. AR window ORR was 93% (CR 57%). MRD negativity was achieved in 18% (7/38) post AR window, and 94% (34/36) post RDHAOx. 43 patients remained evaluable for response after R-DHAOX and pre-maintenance with 35 (80%) who underwent ASCT and 38 (86%) who commenced AR maintenance; 7 remain on maintenance. Response in specific subgroups will be reported at the meeting. At data lock, 35 pts (81%) experienced ≥1 G3+ adverse events during induction or maintenance phases, most common were neutropenia (58% of pts), febrile neutropenia (27%), thrombocytopenia (25%), diarrhea (14%). No G5 treatment-related events have occurred to date. There have been 22 SAEs, most common were COVID (3), febrile neutropenia (2) and fever (2). 5 deaths have been reported: 4 in pts with progressive disease and 1 COVID pneumonitis during AR maintenance. After a median follow up of 22 months (range 17-28m), the 2-year OS was 89%.
Analysis of survival, quality of life and biomarkers will be reported with more mature follow up.
Conclusion
AR delivered in a sandwich approach is active and safe. An AR window yields a high ORR and compared to historical studies, improves post-chemo induction CR rates and MRD negativity. A telehealth model allowed rapid recruitment in a rare cancer.
OffLabel Disclosure:
Hawkes:Gilead: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: Travel Expenses, Research Funding, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Other; Regeneron: Speakers Bureau; Antengene: Membership on an entity's Board of Directors or advisory committees; Specialised Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Other; Merck Sharpe & Dohme: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck KgA: Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding. Cheah:F. Hoffmann-La Roche Ltd, Janssen, Gilead, AstraZeneca, Lilly, TG therapeutics, Beigene, Novartis, Menarini, Daizai, Abbvie, Genmab. BMS: Consultancy; F. Hoffmann-La Roche Ltd, Janssen, Gilead, AstraZeneca, Lilly, TG therapeutics, Beigene, Novartis, Menarini, Daizai, Abbvie, Genmab. BMS: Honoraria; BMS, F. Hoffmann-La Roche Ltd, Abbvie; MSD, Lilly: Research Funding. Wight:Otsuka: Consultancy, Honoraria; Merck Sharpe Dohme: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel expenses; Abbvie: Consultancy, Honoraria; MDI: Consultancy, Honoraria; Beigene: Consultancy, Honoraria. Ku:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees. Opat:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Research Funding; Merck Sharpe & Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Antengene: Membership on an entity's Board of Directors or advisory committees; Janssen Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Honoraria, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Cochrane:Janssen-Cilag: Speakers Bureau; Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Beigene: Research Funding. Lee:Takeda: Honoraria; Beigene: Honoraria; Gilead: Other: Tavel expenses. Yeh:Astellas: Speakers Bureau. Barraclough:Roche: Honoraria; Gilead: Consultancy, Honoraria.
Acalabrutinib for mantle cell lymphoma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal